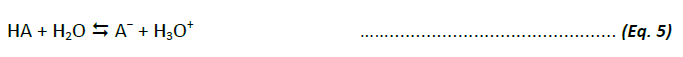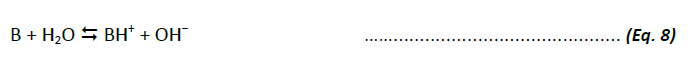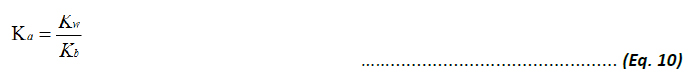Definition
An acid (HA) is a substance that dissociates in water, releasing a hydrogen ion (H+) and a conjugate base (A-). According to Arrhenius, the reaction of the acid-ionisation can be written as:
The value of the equilibrium constant for this dissociation reaction (Ka) is a quantitative measure of the strength of the acid in aqueous solution. In the equilibrium state, the Ka is defined as the quotient of the equilibrium concentrations of the dissociated ions and the free, undissociated acid (in mol/l):
Because the Ka value may span several orders of magnitude, it is usually expressed as a negative decadal logarithm, i.e. pKa = –log10 Ka. The larger the pKa value, the smaller the extent of dissociation of the acid. Acids with a pKa value of about -2 are considered to be strong acids, pKa values of 12 indicate weak acids or strong bases. Strong acids with pKa values of less than -2 are almost completely dissociated (> 99%) in aqueous solution at pH 0. The pKa can be calculated:
The concentration of organic acids in the dissociated (A-) and free (HA) forms are equal when pH = pKa, and the ratio of A- to HA increases by an order of magnitude for each unit of pH above pKa. A comparison of the pKa of an organic acid with the pH of the aqueous system of concern reveals the importance of acid dissociation of the organic compound in determining the environmental distribution.
Polyprotic acids are acids that can donate more than one proton to a base. The equilibrium constants for the successive dissociation reactions are referred to as Ka1 for the first proton, Ka2 for the second proton, etc. When the difference between successive pKa values is . 4, each species can be considered as an acid in its own right.
The acid-base behaviour of weakly basic organic compounds (B) is defined by the base dissociation constant, Kb, analogously:
For convenience, it is preferable to address the behaviour of weak bases in terms of the Ka or pKa values:
The Ka for the conjugate acid, BH+, and Kb for the base, B, are related by the auto-dissociation constant of water Kw:
The acid strength can vary over about 50 orders of magnitude. In aqueous media however, the range of interest is restricted to acids with pKa values of 0 to 14. At normal environmental pH (5 - 8), the range of acidities that are of concern is even more restricted, i.e. pKa range of 3 to 10. If an organic species has a pKa outside these limits, it is expected to be either completely (> 99%) dissociated (pKa of organic acid < 3) or completely undissociated (pKa of conjugate acid > 10) in an aqueous environment.